X-chromosome inactivation is a fascinating cellular process that serves as a crucial mechanism in gene dosage balance between males and females. In females, where two X chromosomes are present, one is silenced to prevent an overexpression of X-linked genes. This intricate phenomenon has been the focus of extensive research, with Jeannie T. Lee from Harvard Medical School leading pioneering studies that shed light on the underlying mechanisms. Her recent findings could have significant implications for treating genetic disorders such as Fragile X Syndrome and Rett Syndrome. By understanding how X-chromosome inactivation operates, researchers hope to unlock new avenues for therapies that could improve the lives of those affected by these conditions.
The process of silencing one of the two X chromosomes in female cells is known as X-inactivation, a crucial event that ensures genetic balance in different sexes. This mechanism prevents females from expressing excessive genes associated with the X chromosome, which could lead to various health issues. Researchers like Jeannie T. Lee, affiliated with Harvard Medical School, have devoted years to unraveling the complexities of this genetic regulation. Recent breakthroughs suggest that manipulating X-inactivation may offer new therapeutic options for individuals suffering from disorders like Fragile X Syndrome and Rett Syndrome. By exploring the dynamics of this genetic silence, scientists are hopeful for advancements that could lead to innovative treatments for such genetic disorders.
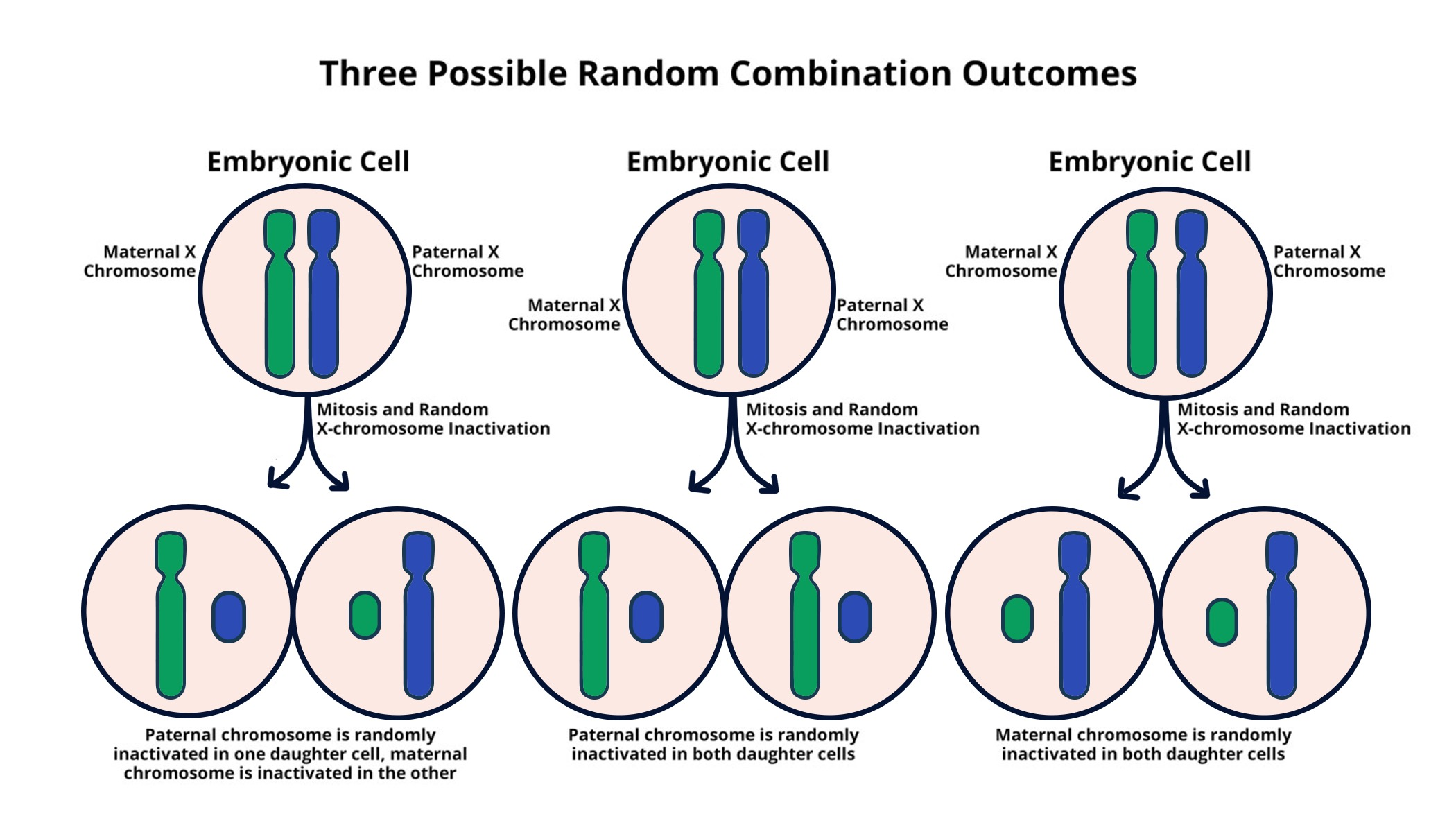
Understanding X-Chromosome Inactivation
X-chromosome inactivation is a crucial biological mechanism that allows female cells to balance gene expression between their two X chromosomes. Unlike males, who possess a single X chromosome, females must inactivate one copy to avoid an overexpression of genes that could lead to cellular dysfunction. This process, orchestrated by a unique RNA molecule known as Xist, plays a fundamental role in maintaining genetic stability. Jeannie T. Lee’s groundbreaking research at Harvard Medical School has shed light on the complexities of how this inactivation process occurs and its implications for genetic disorders.
The intricacies of X-chromosome inactivation involve a gelatinous substance that envelops the chromosomes, creating a protective layer. Lee and her colleagues have discovered that this ‘Jell-O-like’ material acts as a barrier that allows for selective accessibility of certain genes. By understanding how Xist interacts with this substance to inactivate the X chromosome, researchers can potentially unlock new therapeutic avenues for treating genetic disorders like Fragile X Syndrome and Rett Syndrome.
Potential Therapies for Fragile X and Rett Syndromes
Fragile X Syndrome and Rett Syndrome are two significant neurodevelopmental disorders linked to mutations on the X chromosome. Fragile X is known to cause cognitive impairment and developmental delays, while Rett syndrome primarily affects females and leads to a regression in motor and cognitive skills. Jeannie T. Lee’s research into X-chromosome inactivation posits that restoring function to the inactivated X chromosome may present a viable treatment strategy for these conditions. By freeing X-linked genes from silencing, there is potential to utilize the healthy versions of the genes that are typically rendered inactive.
The promise of using compounds developed from Lee’s lab within clinical trials represents a revolutionary shift in how we approach these genetic disorders. Through the optimization of strategies to unsilence X-linked genes, researchers hope to mitigate the effects of these conditions. Importantly, the goal is to target only the mutated genes without disrupting the function of healthy genes, minimizing potential side effects. This approach not only serves females but may also have significant implications for males affected by similar mutations on their single X chromosome.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important in genetic disorders like Fragile X Syndrome and Rett Syndrome?
X-chromosome inactivation (XCI) is a crucial biological process in females that ensures one of the two X chromosomes is silenced. This is important in genetic disorders such as Fragile X Syndrome and Rett Syndrome, which are linked to mutations on the X chromosome. By understanding XCI, researchers like Jeannie T. Lee from Harvard Medical School aim to develop therapies that could potentially unsilence mutated genes associated with these disorders.
How does the research by Jeannie T. Lee contribute to understanding X-chromosome inactivation?
Jeannie T. Lee’s research at Harvard Medical School has significantly advanced our understanding of X-chromosome inactivation by revealing the molecular mechanisms behind how this process occurs. Her studies indicate that a gelatinous substance surrounding chromosomes facilitates XCI by allowing the Xist RNA molecule to alter its properties, making the X chromosome inactive. This discovery is vital for developing treatments for conditions like Fragile X Syndrome and Rett Syndrome.
Can X-chromosome inactivation be targeted for potential treatments of genetic disorders?
Yes, targeting X-chromosome inactivation holds promise for treating genetic disorders like Fragile X Syndrome and Rett Syndrome. Jeannie T. Lee’s lab has shown that unsilencing the inactivated X chromosome can restore the function of mutated genes without affecting healthy ones. This presents a therapeutic avenue with minimal side effects, opening the door for innovative treatments for patients with these conditions.
What role does the RNA molecule Xist play in X-chromosome inactivation?
The RNA molecule Xist plays a critical role in X-chromosome inactivation by coating the X chromosome and changing the properties of the surrounding gelatinous substance, which leads to the chromosome’s silencing. Jeannie T. Lee’s research highlights how Xist interacts with this ‘Jell-O-like’ substance to facilitate the inactivation process, which is crucial for understanding the genetic mechanisms underlying disorders like Fragile X Syndrome.
How can understanding X-chromosome inactivation help in the treatment of disorders linked to the X chromosome?
Understanding X-chromosome inactivation can aid in developing treatments for disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome, by providing insights into how to unsilence the inactivated X chromosome. Research led by Jeannie T. Lee suggests that therapies could reactivate healthy genes buried within the inactive X, potentially curing or alleviating symptoms of these genetic disorders.
| Key Points |
|---|
| X-chromosome inactivation (XCI) resolves the gene dosage imbalance between males and females, as females have two X chromosomes, while males have one. |
| Jeannie T. Lee’s lab has been pivotal in discovering the mechanisms behind X-chromosome inactivation, which has implications for treating certain genetic disorders. |
| The process involves a gelatinous substance, likened to Jell-O, which helps orchestrate the inactivation of one of the X chromosomes in females. |
| Xist RNA plays a crucial role in the inactivation process by altering the physical properties of the coating around the X chromosome. |
| Understanding XCI may lead to therapies for X-linked disorders like Fragile X Syndrome and Rett Syndrome by ‘unsilencing’ mutated genes. |
| Lee’s ongoing research aims to optimize potential treatments and commence clinical trials in the coming years. |
Summary
X-chromosome inactivation (XCI) is a crucial biological mechanism that ensures females, who have two X chromosomes, do not express double the amount of genes compared to males. This process, explored extensively by Jeannie T. Lee’s lab, involves the intricate interplay of RNA and a gelatinous substance that modulates gene expression on the X chromosome. The implications of understanding XCI are significant, as it opens pathways to novel therapies for genetic disorders such as Fragile X Syndrome and Rett Syndrome. By deciphering the mechanisms behind XCI, researchers like Lee are not only answering fundamental biological questions but also paving the way for innovative treatments that could benefit countless individuals affected by X-linked disorders.